|
Virus seed stock
The seed vaccine orf virus (Hou/SA/97) was selected from two field orf viruses which caused fulminant orf outbreaks in sheep and goats in 1995 and 1997. They were obtained from our collection, which was stored at −86 °C in glycerol buffered saline at pH 7.4. Characterisation studies and selection of the seed virus followed the guidelines of the OIE (OIE 2008), which have previously been reported (Housawi 2008).In the present study, scab material containing the seed virus was made up as a 30% suspension (w/v) in phosphate buffered saline (PBS) pH 7.4. Following centrifugation at 377 × g for 15 min, the supernatant fluid was collected and antibiotics (procured through the Saudi branch of Sigma-Aldrich) were added at a concentration of 100 IU/mL penicillin, 1 mg/mL streptomycin and 50 IU/mL mycostatin. The supernatant fluid was used to inoculate monolayers of Vero cell culture, as described by Housawi et al. (1991). When the cytopathic effect (CPE) involved 90% of the cell monolayer, the cells were harvested and stored in 0.5 mL aliquots at −86 °C.
Study population
Orf-seronegative indigenous Noemi sheep, aged six months old, were used in the study. They were procured from a farm with no history of orf infection and were kept in complete isolation from other animals to avoid natural orf infection. They were provided with feed and water until used in the experiments.The ethical approval to use the sheep was provided by the Ethical Committee of the Faculty of Veterinary Medicine and Animal Husbandry, King Faisal University, Saudi Arabia.
Vaccines
Three types of live orf vaccines were prepared, namely, a live scab vaccine (LSV), Vero cell culture passage 20 vaccine (P20V) and Vero passage 75 vaccine (P75V). For preparation of the live scab vaccine (LSV), the ‘master seed virus’ was inoculated into five sheep, as described by Housawi et al. (1993). The sheep were observed daily until the development of the orf lesions. The scab material was collected and stored at −86 °C until used for preparation of the LSV vaccine. The scab material was made up as a 50% (w/v) suspension in PBS pH 7.4, processed as described for
the virus seed stock and stored at −86 °C until titrated in monolayers of Vero cells
(Housawi et al. 1991). The tissue culture infective dose 50 (TCID50)
was calculated following the method of Read and Muench (1938). The virus suspension was adjusted to contain 106 TCID50/mL in PBS-glycerol (50% v/v). The formulated vaccine was stored at 4 ºC. To prepare the P20V vaccine, the seed virus was serially passaged in monolayers of the Vero monkey kidney cell culture (Housawi et al. 1993). The 20th passage (P20V) was harvested and titrated in Vero cell monolayers as above. Aluminium hydroxide gel (Sigma-Aldrich, Saudi branch) was then added at a concentration of 1.6 mg/mL and the final titre was adjusted to 106 TCID50 /mL. The vaccine was stored at 4 °C until used. The same procedure which was used for preparation of the P20V above was adopted for preparation and formulation of the P75V vaccine, except that the seed virus was serially passaged 75 times in Vero cell culture.
The challenge virus
Scab material from the experimentally infected sheep as described above, was made into a 50% (w/v) suspension in PBS pH 7.4, centrifuged at 377 × g for 15 min, and the supernatant fluid was collected,
processed as before and 0.5 mL aliquots were stored at −86 °C until used in the challenge experiments.
Study design
The experimental sheep were divided into three groups
(n = 30) and each group was allocated to one of the respective vaccines. A further fifteen sheep were kept as unvaccinated controls in the challenge experiments.The group of 30 sheep used in the LSV vaccine experiments was subdivided into two equal groups of 15 sheep, A and B. Both groups were subdivided into three subgroups of five sheep each, A1, A2 and A3, and B1, B2 and B3. The three A-subgroups received only a primary dose of the LSV, whilst the B-subgroups received the primary dose and a booster dose after a month. The same procedure of creating groups and subgroups was followed for the P20V and P75V vaccines, with the same numbers of sheep for each group. The designations for the P20V groups were D and E, and those for the P75V subgroups were G and F. The subgroups were D1, D2 and D3; E1, E2 and E3; F1, F2 and F3; and G1, G2 and G3. Group D received only the primary P20V vaccine dose and Group E received the P20V vaccine dose and a booster dose a month later. Group G received only the primary P75V vaccine dose and Group F the primary P75V vaccine dose followed by a booster dose a month later. The 15 control sheep were also subdivided into three equal sub-groups and each sub-group was used as a control at a challenging point. Table 1 shows the vaccination schedule. All sheep of all the subgroups, except the controls, were vaccinated by single scarification (3 cm – 4 cm) on the inner thigh of the right hind limb, and 40 µL of the vaccine suspension was applied. The vaccinated sheep were observed daily and clinical changes at the sites of vaccination were recorded (Nettleton et al. 1996). Each of the sheep of all sub-groups, whether they received the primary dose only or the primary dose and a booster or were the controls, were challenged and infected in the manner described above. To evaluate the degree of protection in each challenged sheep (Nettleton et al. 1996), daily clinical observations were performed on the sites of scarification from the first day following scarification until the scabs had dropped. Accordingly, the mean healing time (MHT) was calculated. Each member of the research team (4 members) took daily readings and the mean was calculated. The criterion for complete healing was judged by presence of a smooth skin surface after the scabs had dropped. Before complete healing, the scab is usually strongly adherent to the lesion and its forcible removal at the inner thigh of the sheep is not easy. Forcible mechanical removal of a scab leads to abrasion and possible bleeding, which can easily be seen. In this study, none of the scabs in the experimental sheep was exposed to forcible mechanical detachment before complete healing and shedding. The mean reduction time (MRT) per cent, of the orf lesions in the challenged sheep,
as compared to the unvaccinated control sheep, was calculated by the following formula: 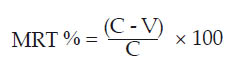 [Eqn 1] [Eqn 1]
where MRT % is the mean reduction time %, C is the mean healing time (days) in the unvaccinated control sheep and V is the mean healing time (days) in the vaccinated sheep.
The ELISA
The reference orf antigen used in the ELISA was provided by the Moredun Institute, UK. It was used as 1% Nonindet P-40 orf specific extract (Nettleton et al 1996). The specific reference sheep anti-orf serum, the non-immune sheep serum collected from orf-free sheep and the mock lamb muscle antigen for ELISA were also provided by the Moredun Institute, UK. The test sheep sera were collected weekly from each sheep subgroup following the primary vaccination or booster. Sera were also collected weekly from the sheep following each challenge (after 6, 12 and 18 months respectively). The sera were inactivated at 56 ºC for 30 min and stored at −20 ºC until used in the ELISA. An indirect ELISA was employed for the detection of humoral antibodies using the above-mentioned reagents. All volumes of reactants were 50 µL per well, incubations at all stages were made at 37 ºC, (except for the substrate which was incubated at room temperature 22 ºC). Washing was by flooding and emptying the wells three times with PBS-Tween (0.01% Tween 20, Sigma-Aldrich, Saudi branch). The ELISA plates were coated with the reference orf antigen, incubated for two hours and washed. The test sera were diluted as required in PBS-T + 2% Ovalbumin (Sigma-Aldrich, Saudi branch) and incubated for one hour and the plates were washed. Donkey anti-sheep IgG conjugated to horseradish peroxidase (Sigma-Aldrich, Saudi branch) was added following instructions of the manufacturers, incubated for one hour and washed. The substrate was prepared by adding a 30 mg tablet of orthophenyl diamine (Sigma-Aldrich, Saudi branch) to 75 mL distilled water followed by 40 µL of hydrogen peroxide just before use. The substrate was added and the plates were incubated at room temperature for 10 min in the dark. The reaction was stopped by adding 1 M sulphuric acid and plates were read in an ELISA Reader (Dynatec Co.) at 450 nm and the results were interpreted as instructed by the manufacturers.
Statistical analysis
Following each challenge point (after 6, 12 and 18 months), the ANOVA, in conjunction with a post hoc test, was used to evaluate the difference
in protection between all the vaccinated groups and also between the vaccinated groups and the unvaccinated controls.
|
TABLE 1: Vaccination schedule of the experimental sheep.
|
|
TABLE 2: Challenging schedule of the vaccinated sheep and the unvaccinated
controls.
|
Ethical considerations
Ethical approval for use of the experimental sheep and all protocols in this study were obtained from the Ethical
Committee of the Faculty of Veterinary Medicine & Animal Husbandry, King Faisal University, Saudi Arabia.
Regardless of the vaccine type, typical stages of orf infection, viz., erythema, papule, pustule and scab formation, were seen following primary vaccination or the booster dose in all vaccinated sheep. However, some overlap was observed between the different stages of infection. Table 3a shows the clinical changes at the site of vaccination following primary vaccination. Table 3b shows that, with all three types of vaccines, the duration of healing following the booster dose was shorter than that following the primary dose only. Tables 4, 5 and 6 show the ANOVA and post hoc statistical analysis results of the different groups receiving the different vaccines and challenged after 6, 12 and 18 months.
The results indicate highly significant differences between the groups at the 0.05 level and 95% confidence interval (p < 0.05). Highly significant differences
(p < 0.05) were also obtained between the vaccinated and unvaccinated control groups of sheep. Figure 1 illustrates the MRT % values, following each of the 3 challenges in the vaccinated sheep. Following the six months’ challenge of the groups that received one dose of the relevant vaccine, the MRT % value was highest for the P20V (33.3%), followed by that for the P75V (30%) and the LSV group (16.7%) respectively. Following the 12 months’ challenge, the highest MRT % value was scored for the sheep that received the P20V (30%), followed by those that received the LSV (26.7%) and the lowest was scored by the P75V (16.7%) group. Following the 18 months’ challenge, the MRT % values were 33.3%, 16.7% and 13.3% for the P20V, LSV and P75V respectively. Figure 2 illustrates the MRT % values for the boostered sheep at each challenge point. At the 6 months’ challenge, the MRT % values were 43.3%, 33.3% and 30% for the P20V, LSV and the P75V respectively. At the challenge point after 12 months, the MRT % values were 36.7% for the P20V and 26.7% for both the LSV and P75V. Following the challenge point at 18 months, the MRT % values were 33.3%, 16.7% and 13.3% for the P20V, LSV and P75V respectively. Figure 3 represents a typical pattern of ELISA values reflecting the serum antibody levels following challenges (as exemplified by the P20V vaccinated sheep following the 6 months’ challenge). The optical density (OD) values shown on the y-axis represent net values obtained by subtracting the background reactivity of the pre-immune serum. All samples were measured in duplicate. As seen in Figure 3, the mean OD value at the day of challenge (day zero) was high (2.93 ± 0.12) then dropped to 2.02 ± 0.15 on day 7 post challenge (PC), (most probably due to neutralisation by the challenge virus). On day 14 PC, the OD level rose to 2.83 ± 0.13, then there was a slight drop by day 21 PC (2.52 ± 0.15) and it remained almost at that level until day 28 PC (2.51 ± 0.14). The challenged unvaccinated control sheep were ELISA seronegative at the time of challenge but seroconverted after challenge. The OD values started rising from day 7 PC to reach a high level by day 28 PC.
|
TABLE 3a: Mean clinical changes at the site of scarification after primary vaccination.
|
|
TABLE 3b: Mean clinical changes at the site of scarification after the booster dose.
|
|
TABLE 4: ANOVA results for all the vaccinated and unvaccinated sheep groups
following the 6 month challenge.
|
|
TABLE 5: ANOVA results for all the vaccinated and unvaccinated sheep groups
following the 12 month challenge.
|
|
TABLE 6: ANOVA results for all the vaccinated and unvaccinated sheep groups
following the 18 month challenge.
|
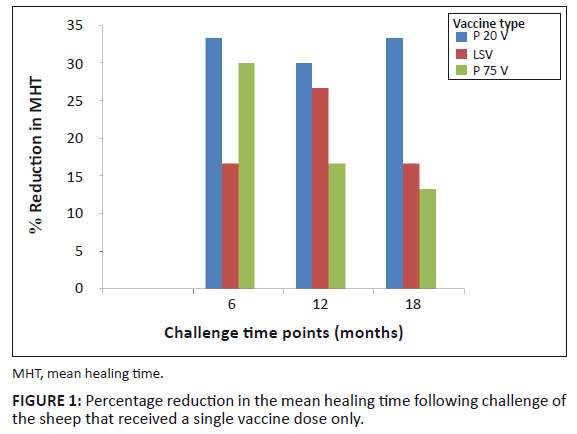 |
FIGURE 1: Percentage reduction in the mean healing time following challenge of
the sheep that received a single vaccine dose only.
|
|
 |
FIGURE 2: Percentage reduction in the mean healing time following challenge of
the sheep that received the booster vaccine dose.
|
|
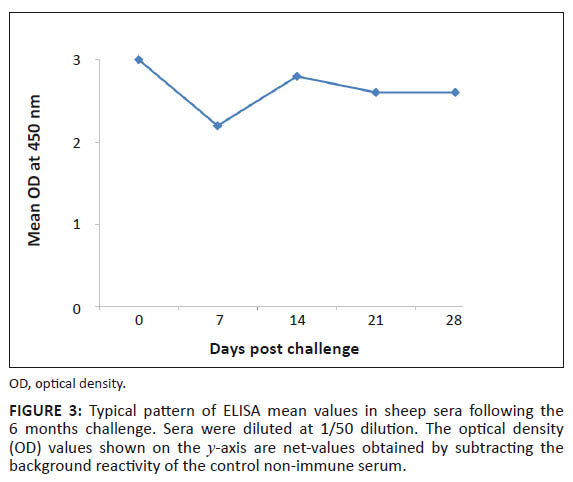 |
FIGURE 3: Typical pattern of ELISA mean values in sheep sera following the
6 months challenge. Sera were diluted at 1/50 dilution. The optical density
(OD) values shown on the y-axis are net-values obtained by subtracting the
background reactivity of the control non-immune serum.
|
|
Unlike other animal viral vaccines, which may confer full protection to the vaccinated animal, orf vaccines do not give 100% protection. Therefore, the main purpose behind orf vaccination is to protect vaccinated animals from the severe effects of the field virus and to offer appreciable reduction in the duration of the clinical disease. The present study was undertaken to develop a local efficacious vaccine to be used in Saudi Arabia, where orf infection is widespread. Accordingly, three types of live vaccines were developed from a local field orf virus so as to choose the most efficacious to be recommended for use in the field. To the best of the authors’ knowledge, no similar comparative study, utilising three types of vaccine prepared from the same virus strain, has been conducted in the Middle East nor in the developing world.
Each of the developed vaccines gave clinical responses at the site of vaccination (indicating viability), induced production of humoral antibodies and conferred some degree of protection. To evaluate the ability of the vaccine to minimise the time of healing following challenge, a simple formula was developed in this study to calculate the rate of mean reduction in the healing time (MRT %) following challenge. This exercise enabled direct comparison of the efficacy of the three types of vaccines.
Comparing the efficacy of the three types of vaccines, it could be seen that P20V gave the best results. It induced highly significant reduction in disease duration following the three challenge points. It is clear that the twenty passages of the orf virus in Vero cell culture were satisfactory for the production of a safe and efficacious P20V orf vaccine under our local conditions. It is rather difficult to explain why this occurs, but it is likely that at this passage level the virus has lost its high virulence but can maintain its immunogenicity to confer good protection. Generally speaking, the results for the three types of vaccine indicate that protection in the boosted sheep was better than in those that received only one dose of the vaccine, although no improvement was seen in the P75V results. Published data indicate that orf protection is predominantly cell mediated. However, humoral antibodies are also vital in the activation of the killer cell (antibody dependent cytotoxic cell – ADCC), which is an important branch of the cell-mediated immunity. The present study has illustrated that the three types of vaccine induced the production of humoral antibodies following primary vaccination of the sheep and resulted in an amnesic response following the booster dose.
This study involved the development of three different orf vaccines from the same virus isolate, for the first time in the Middle East and probably elsewhere.Two of the vaccines were prepared by passaging at different levels in Vero cell culture; and the third was used as a virulent virus in glycerine buffer saline. The P20V was found to be the most efficacious of the three vaccines, and is recommended for use in the field in Saudi Arabia. In the present study, a novel formula was also developed to calculate the healing time as a percentage of that seen in control animals instead of scoring it in days.
This will enable researchers in the field of orf vaccinology to compare their results directly.
The authors would like to thank King Abdulaziz City for Science and Technology for Grant Number AT-19-6 and for their ever continuous encouragement. We also would like to thank Dr P. Nettleton for provision of the reference ELISA antigen and antiserum. The technical assistance
of Mr A. Khars and the kind help of Dr Nahla Khames in the statistical analysis are appreciated.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this paper.
Authors’ contributions
F.M.H. (King Faisal University) was the principal investigator. All authors participated in the project design and brain storming discussions. E.M.A. (King Faisal University) prepared the vaccines. E.M.A. (King Faisal University) and F.M.H. (King Faisal University) performed the ELISA experiments. A.A.G. (King Faisal University) was the pathologist of the team. He inoculated the sheep with the different vaccines. A.A.G. (King Faisal University), E.M.A. (King Faisal University), F.M.H. (King Faisal University) and A.I.A. (King Faisal University) participated in reading of the vaccination & challenge results and subsequent follow-up. E.M.A. (King Faisal University), F.M.H. (King Faisal University), A.A.G. (King Faisal University) and A.I.A.
(King Faisal University) wrote the manuscript. E.M.A. (King Faisal University) is the corresponding author.
Abuelzein, E. & Housawi, F., 1997, ‘Severe long-lasting contagious ecthyma infection
in a goat’s kid’, Journal of Veterinary Medicine Series B 44, 561–564.
http://dx.doi.org/10.1111/j.1439-0450.1997.tb01008.x
Abuelzein, E. & Housawi, F., 2009, ‘Drastic cutaneous multi-focal orf infection in goats,
causing severe dysfunctioning’, Revue Scientifique et Technique (OIE) 28, 1025–1029.
PMid:20462159
Andrewes, C. & Horstman, D., 1949, ‘The susceptibility of viruses to ethy1 ether’,
Journal of General Microbiology 3, 290–292.
PMid:18149942
Gameel, A., Abuelzein, E., Housawi, F., Agib, A. & Ibrahim, A., 1995, ‘Clinico-pathological observations
on naturally occurring contagious ecthyma in lambs in Saudi Arabia’, Revue d’Elevage et de Médecine
Vétérinaire des Pays Tropicaux 48, 233–235.
PMid:8745744
Housawi, F., 2008, ‘Characterization of candidate seed orf viruses to be used as vaccine in sheep and goats in
Saudi Arabia’, Scientific Journal King Faisal University (Basic Sciences) 9, 137–146. Housawi, F. & Abuelzein, E., 1991, ‘Orf infection following ear tagging in goats’,
Revue d’Elevage et de Médecine Vétérinaire des Pays Tropicaux 44, 277–278. Housawi, F., Abuelzein, E., Amin, M. & Al Afaleq, A., 1991, ‘Orf infection in sheep and goats
in Saudi Arabia’, Veterinary Record 128, 550–551.
http://dx.doi.org/10.1136/vr.128.23.550,
PMid:1909477
Housawi, F., Abuelzein, E., Al Afaleq, A. & Amin, M., 1992, ‘Serosurveillance for orf antibodies in sheep and goats in Saudi Arabia employing the ELISA technique’, Journal of Comparative Pathology 106, 153–158.
http://dx.doi.org/10.1016/0021-9975(92)90044-U
Housawi, F., Abuelzein, E., Gameel, A. & Al Afaleq, A., 1993, ‘A close comparative study on the response of sheep and goats to experimental orf infection’, Journal of Veterinary Medicine Series B 40, 272–282.
http://dx.doi.org/10.1111/j.1439-0450.1993.tb00138.x
Marklew, S., 1995, ‘Assessment of cell-culture grown on virus vaccines in sheep’, in M. Schwyzer (ed.),
Immunology Viral Infections: Proceedings of the 3rd Congress European Society, Veterinary Virology,
September 4–7, 1995, pp. 305–309. Nettleton, P., Brebner, J., Pow, T., Gilray, J., Bell, G. & Reid, H., 1996, ‘Tissue culture propagated orf
virus vaccine protects lambs from orf virus challenge’, Veterinary Record 138, 184–186.
http://dx.doi.org/10.1136/vr.138.8.184,
PMid:8677620
Office International Epizooties (OIE), 2008, ‘Bovine Viral Diarrhoea’,
Manual of Standards for Diagnostic Tests and Vaccines , Chapter X.5, p. 18, from
http://www.oie.int
Reed, L. & Muench, G., 1938, ‘A simple method of estimating fifty percent point’,
American Journal of Hygiene 27, 493. Robinson, A. & Balassu, T., 1981, ‘Contagious pustular dermatitis (orf)’,
Veterinary Bulletin 51, 771–782. Talhouk, R. & Elzein, E., 1986, ‘Characterization of a cell culture adapted goat pox virus’,
Journal of Veterinary Medicine Series B 33, 543–551.
http://dx.doi.org/10.1111/j.1439-0450.1986.tb00066.x
|
