|
Wound healing is a fundamental response to tissue injury and several natural products have been shown to accelerate the healing process. The present study was undertaken to determine the safety and efficacy of the topical treatment of acetone leaf extracts of Combretum imberbe, Combretum nelsonii, Combretum albopuntactum and Terminalia sericea based on their in vitro antimicrobial activity. Four circular full-thickness skin wounds were made on the backs of eight anaesthetised Wistar rats using aseptic techniques. The treatments were administrated topically using 10% and 20% concentrations of each extract in aqueous cream in separate treatments. Indications of erythema, exudate, crust formation, swelling and ulceration were used to determine the wound healing process. All of the wounds closed completely within 17 days. Throughout the experiment, a subcutaneous probe was used to determine that the body temperature and body weight of the rats were within the normal range. C. imberbe and C. nelsonii extracts accelerated wound healing, but there was no significant difference in wound contraction using 10% and 20% concentrations of the extracts in cream. The results also showed the potential usefulness of this model to measure accelerating wound healing. The extracts could perhaps overcome defects associated with healing failure in chronic wounds and prevent secondary bacterial and fungal infections.
The wound healing process involves a highly coordinated cascade of cellular and immunological responses over a period of time. The first phase (also known as the coagulation phase) occurs immediately after the traumatic injury and serves to seal the wound with a fibrin and platelet plug and to initiate the inflammatory process. Cytokines produced by the platelets cause leukocytes from the blood circulation to amass at the site of injury. The predominant cell type in the first three days after injury is neutrophils, which phagocytose microbes and foreign debris. Thereafter, activated macrophages at the wound site assist in wound repair by the release of proteases for wound debridement and by the phagocytosis of microbes, cell debris and damaged neutrophils. Together with other cells, macrophages in the area produce various cytokines, chemokines and growth factors, which, in turn, cause the influx and activation of more macrophages, as well as the stimulation of the adaptive immune response (Waldorf & Fewkes 1995). Granulation tissue forms 2–3 days after the injury and consists predominantly of fibroblasts. Immediately following the injury and during granulation, keratinocytes migrate into the matrix and proliferate under the influence of growth factors produced by macrophages, keratinocytes and fibroblasts; while, at the same time, neovasculisation occurs. In the second week after the injury, the wound contracts as a result of the activity of adapted fibroblasts known as myofibroblasts. Collagen production and remodelling continues with scar formation (Tsirogianni, Moutsopoulous & Moutsoupoulos 2006). Injuries are common in humans, especially in environments where there is a high prevalence of violent acts and accidents. Owing to poor or delayed wound management, these wounds often become infected with either commensal or environmental microorganisms, or both. Infected wounds heal more slowly, re-epithelialisation is more prolonged and there is a risk of systemic infection (Priya et al. 2002). People in rural areas in South Africa often use medicinal plants for the initial treatment of wounds as they are easily accessible and cheap. In these areas, the most common way to treat wounds with such plants is by using a decoction of one or more of the plants as a wash. This is followed by the application of a powder made from the plants directly onto the wound, after which the wound is covered by a bandage. The plant material is sometimes carbonised before pounding into a powder. An emulsion of a powder prepared from various parts of the plant and oil from the seeds of Lannea microcarpa (Anacardiaceae), or butter of Butyrospermum parkii (Sapotaceae) can also be used, as well as juice obtained by squeezing or heating the plant leaves or roots. These treatments are usually repeated every day until the wounds are healed. Bandages most often consist of whole, fresh leaves, or a strip of cotton cloth (Inngjerdingen et al. 2004). The process of wound healing is promoted by several plant products (Suguma, Chandrakasan & Joseph 1999), which contain active principles such as triterpenes, alkaloids, flavonoids (Sharma et al. 1990) and biomolecules (Chithra, Sajithlal & Chandraksan 1995). These agents usually influence one or more phases of the healing processes. To date, the wound healing properties of two tropical plants, Centella asiatica (Suguma, Sivakumar & Chandrakasan 1996) and Terminalia chebula (Suguma, Surjeet & Chandrakasan 2002), have been demonstrated in rat models. Many species of the Combretaceae family that are widely distributed in Africa are used in traditional medicine for treating a myriad of conditions including, pneumonia, syphilis, abdominal pains, conjunctivitis, diarrhoea, leprosy, scorpion stings and mumps (Hutchings et al. 1996). Some species of this plant family are also used for wound healing, for example, Hutchings et al. (1996) have reported that the Vhavenda people of northern South Africa use the leaves of Terminalia sericea for the treatment of wounds and menorrhagia, while the bark of this species is used to treat wounds and the roots for diarrhoea, infertility and venereal diseases (Mabogo 1990). Mabogo (1990) has reported that the roots of Combretum molle are used by the Vhavenda for wound healing, while, in Ghana the leaves of this species are used for wound dressing. Other plant species within the Combretaceae family that are used for medicinal purposes include Combretum kraussii, the leaves of which have been used to treat wounds (Hutchings et al. 1996) and Combretum micranthum, the leaves and fruits of which can be used for the topical treatment of wounds (Iwu 1993; Oliver-Bever 1986). Van Wyk et al. (1997) have also reported that T. sericea and C. kraussii are used for the topical treatment of wounds. Acetone extracts of Combretum imberbe, Combretum nelsonii Combretum albopuntactum and T. sericea have antibacterial
properties that work against Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis and Escherichia
coli (Eloff 1999). In earlier studies, we found that Combretum and Terminalia extracts demonstrated remarkable activity
against Candida albicans, Cryptococcus neoformans, Microsporum canis, Sporothrix schenckii and Aspergillus fumigatus,
having a minimum inhibitory concentration (MIC) as low as 0.02 mg/mL Mosmann 1983). This study was conducted by applying the extracts of C. imberbe, C. nelsonii, C. albopuntactum and T. sericea to wounds created in Wistar rats to determine if these extracts induced any skin irritation, damage or other negative effects.
This research was approved by the Research and Animal Use and Care Committee of the University of Pretoria, South Africa (VI 010/05 approval number).
Plant collection
Leaves were collected in the summer of 2003 from four species of trees of the Combretaceae family in the Lowveld National Botanical Gardens in Nelspruit, South Africa. Voucher specimens in the garden herbarium and tree labels verified the identity of the plants. The selection of the species was based on their high antifungal activity which had been demonstrated in previous in vitro studies (Masoko et al. 2005, 2007) and low in vitro toxicity (Masoko, 2006). Leaves were collected from the four species belonging to different sections of the genus (Carr 1988), C. imberbe Wawra (Hypocrateropsis Engl. & Diels), C. nelsonii Duemmer (Angustimarginata Engl. & Diels), C. albopunctactum Suesseng (Ciliatipetala Engl. & Diels) and T. sericea Burch ex DC (Psidioides).
Plant storage
Leaves were separated from the stems and dried at room temperature. Most scientists have tended to use dried material because there are fewer problems associated with the large-scale extraction of dried plants rather than fresh plant material (Eloff 1998a). The dried plants were milled to a fine powder in a Macsalab mill (Model 200 LAB; Eriez, Bramley, South Africa) and stored at room temperature in closed containers in the dark until used.
Extraction procedure
The dried leaves of each species were individually extracted by treating 500 g of finely ground plant material with 5 L of acetone (technical grade; Merck, Johannesburg, South Africa) in big glass containers (Eloff 1998b). The containers and contents were vigorously shaken for 3 h – 5 h in a Labotec model 20.2 shaking machine at high speed. The particulate matter was allowed to sediment and the supernatant was filtered and evaporated using a rotavaporator (R-114; Büchi, New Castle, USA) and decanted into pre-weighed labelled beakers. The process was repeated three times to exhaustively extract the plant material, after which the extracts were combined. The solvent was removed under a stream of air in a fume cupboard at room temperature. The extract was then weighed to quantify the extraction efficiency.
Preparation of extract
Each dried extract was ground to a fine powder with a pestle in a mortar and mixed with an aqueous cream – obtained from Reitzer Pharmaceuticals (Pty) Ltd (Sandton, South Africa) – consisting of distilled water, white petroleum jelly, mineral oil, emulsifying wax and phenoxyethanol to a concentration of either 10% (1 g extract per 10 g cream) or 20% (2 g extract per 10 g cream) and kept at 4 °C until used.
Selection of rats
Ten healthy male Wistar rats weighing 150 g – 200 g were used. The test was conducted using a single gender in order to reducing variability and to minimise the numbers required (Organisation for Economic Co-operation and Development (OECD), 2000). At the commencement of the study, each rat was 8–12 weeks old and the weight variation of animals used did not exceed ± 20% of the mean weight of all previously dosed animals (Anon. 2001).
Housing and feeding conditions
The rats were kept at the University of Pretoria Biomedical Research Centre and housed in separate cages at a temperature of 22 °C (± 2 °C) and relative humidity (50% – 60%) in a light–dark cycle of 12 hours. They were fed a conventional rodent diet obtained from Epol (Pty) Ltd (Randburg, South Africa) and had an unlimited supply of drinking water. Environmental enrichment, for example, bedding (wood wool), was provided to keep the rats occupied. Previous work suggests that the provision of enrichment items, such as wood wool, gives laboratory rats the opportunity to perform exploratory and gnawing activities. This can be used to improve their well-being and to distract them from tampering with dressings (Zhu et al. 2006).
Preparation of animals
The cages of the rats were labelled from one to eight for identification purposes. The rats were kept in their cages for at least five days prior to commencement of the experiment to allow for acclimatisation to the laboratory conditions (Spielmann et al. 1999) and they were handled daily during this period. They were not immunosuppressed.
Wound creation
The hair on the back of each of the rats was removed with electrical clippers and bare areas were disinfected using a solution of 0.5% chlorhexidine in 70% alcohol, which was allowed to dry after application. The rats were anaesthetised with isoflurane (0.01 μg/kg – 0.05 μg/kg). Four evenly spaced wounds were made in the skin within the bare area using a punch to obtain biopsies with a diameter of 6 mm (Simosen, Petersen & Groth 2002). The whole process was carried out in a biosafety class II cabinet to limit infection. A temperature probe (microchip; Identipet, Johannesburg, South Africa) was injected subcutaneously into the rump of each rat, so that the body temperature could be routinely monitored during the course of the experiment without unduly disturbing the animal.
Topical application of aqueous creams
The treatments were applied on separate lesions (A, B, C and D) three times a week as follows: A, no treatment, B, cream only, C and D were 10% and 20% of crude extract, respectively. These lesions were randomly selected and two rats were selected for each plant (i.e. eight rats in total were used). To avoid interference by the rat of mixing of treatment a dressing was applied.
Observations
A blind study was done and the observations for each rat were recorded separately. Every Monday, Wednesday and Friday, at 09:00, each rat was taken out of its cage, anaesthetised with isoflurane, its wound dressing removed and various parameters were measured, including their weight and body temperature; the latter was read electronically via an electronic reader. The lesions on each rat were rated using the following parameters, (1) the presence and type of exudate, (2) erythema, (3) swelling, (4) ulceration and (5) crust formation. The scoring criteria are shown in table 1. Each lesion diameter was also measured in millimeters in the vertical and horizontal planes using the electronic digital calipers. The wounds were then cleaned using clean cotton wool and the treatments were reapplied, after which the wounds were re-dressed and the rat allowed to recover and placed in his cage. The rats were observed individually at least once during the first 30 minutes after treatment and then periodically during the next 24 hours, with special attention being given during the first 4 hours of this period. All the rats were observed daily for any indication of illness and interference with the wound dressing. Clinical signs of disease, included changes in the skin and fur, diarrhoea, lethargy, sleep, weight loss and coma were also checked. Provision was made for the early termination of those rats that were either ill or interfered excessively with the wound dressings. All the rats were euthanised by carbon dioxide inhalation when their wound had fully contracted and upon evidence of scar formation. At necropsy, a pathologist examined the liver, heart, lungs, intestines, lymph nodes and kidneys of the rats for gross abnormalities.
With the exception of Rat 1, no rat in this study lost weight. Rat 1 was observed to have lost weight on Day 3, but by Day 5 its weight was
once again within the normal range (Figure 1). The rats’ temperatures were also within the expected values of 35 °C – 37
to percentages of the original size. Since the results of the two rats which were treated the same were practically identical, the averages of these measurements were used. To find out if treatment accelerated wound healing, the wound sizes were compared to that of the untreated wound. Combretum imberbe extracts (Figure 3a) healed the wound faster than their control, followed by C. nelsonii (Figure 3b). The controls healed faster than the 10% concentration of C. albopunctactum, but slower than the 20% extract of this species (Figure 3c). The 10% concentration and the 20% concentration of the T. sericea extracts, in cream, produced almost similar results and were slightly better than the untreated wound (Figure 3d). The resultant healing was also quantified on the basis of erythema (Figure 4), exudate (Figure 5) and crust formation (Figure 6).
 |
Figure 2: Body temperatures of rats measured for 3 weeks
|
|
 |
Figure 3: Lesion sizes of wounds treated with (a) Combretum imberbe, (b) Combretum
nelsonii, (c) Combretum albopunctactum, (d) Terminalia sericea for 3 weeks
|
|
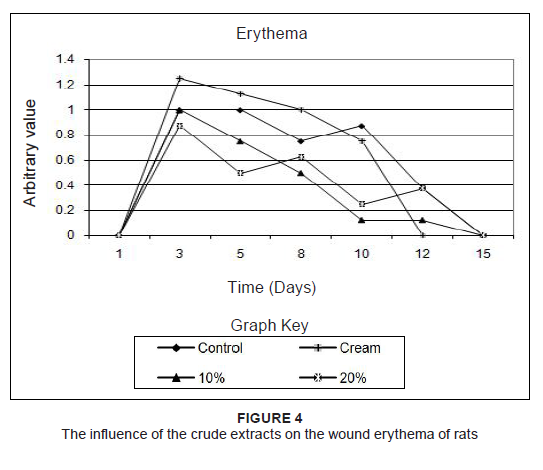 |
Figure 4: The influence of the crude extracts on the wound erythema of rats
|
|
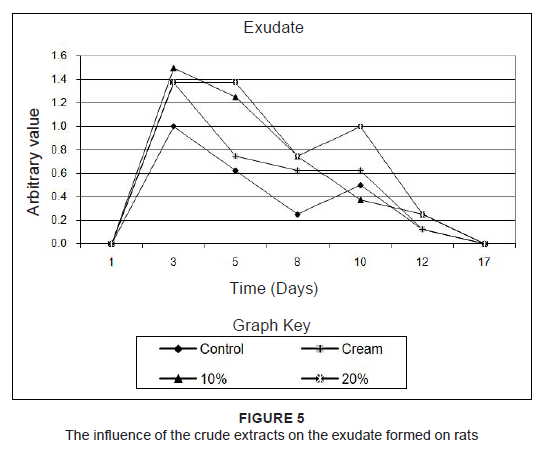 |
Figure 5: The influence of the crude extracts on the exudate formed on rats
|
|
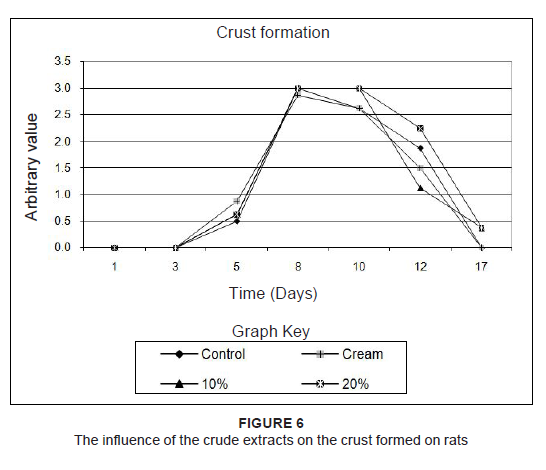 |
Figure 6: The influence of the crude extracts on the crust formed on rats
|
|
Herbal products are usually perceived as ‘safe’ and are often applied to wounds without any in vitro or in vivo toxicity tests being done. This, however, cannot be assumed in all cases. For example, Jatropha curcas L (Euphorbiaceae) was found to have esterases and lipases (Staubmann et al. 1999) and it also has disinfectant, antiparasitic (Fagbenro-Beyioku, Oyibo & Anuforom 1998), antiviral (Matsuse et al. 1999) and molluscicidal activities (Liu et al. 1997). Jathropa curcas was, however, found to be toxic (Makkar, Becker & Schmook 1998). This study was designed using simple in vivo method to determine any adverse effects, as well as to compare the relative effectiveness of acetone leaf extracts of C. nelsonii, C. imberbe, C. albopunctactum and T. sericea on wound healing activity. The in vitro cytotoxicity of acetone extracts of these four species was evaluated on Artemia salina nauplii and Vero monkey kidney cells. The Vero cells were a more sensitive indicator of toxicity (Masoko 2006). At a concentration of 25%, the acetone had no adverse effects on the fungi tested, or on A. salina nauplii and Vero kidney cells. The results on brine shrimps indicated that the four leaf extracts had LC50 values above 20 μg/mL, which is the recommended cut-off point for detecting cytotoxic activity (Geran et al. 1972). The podophyllotoxin toxin standard had LC50 of 7 µg/mL. In the MTT assay, C. imberbe extract had the highest LC50 of 168.6 µg/mL and C. nelsonii had the lowest (75.7 µg/mL) The LC50 for C. albopunctactum and T. sericea were 102.9 µg/mL and 121.7 µg/mL, respectively (Masoko 2006). An LD50 of 30 µg/mL was considered to be non-toxic according to the criteria of the American National Cancer Institute (Suffness & Pezzuto 1990). Although, in practice, local treatments are usually repeated every day until the wounds are healed, it was decided to treat the rats every second day to minimise effects associated with handling and the continuous use of anaesthetics. It was also thought that the plant extracts would retain their antimicrobial activity over that period. Wound contamination was unlikely as the wounds were covered with an occlusive dressing. We found that the rats neither showed any sign of irritation to any of the treatments, including the aqueous cream control, nor did the wounds swell or exhibit noticeable pus formation or ulceration. The wounds were completely healed after 17 days and remained so when the rats were euthanised on Day 21. The created wounds, as well as the contraction and healing of them are depicted in Figure 7. With the exception of Rat 1 and Rat 5, which had transient weight loss and an elevated body temperature, none of the rats showed any signs of clinical disease. It was also not certain whether the illness in these two rats was due to the wound manipulations or a clinical manifestation of interstitial pneumonia that was endemic in these two rats and which was observed at necropsy. We found that the extracts of C. imberbe and C. nelsonii were superior in their wound healing abilities. The wound treated with the C. imberbe extracts (Figure 3a) closed more rapidly than that of the cream and untreated control. In fact, wound closure was slowest in the untreated control. On Day 12, an apparent increase in wound size of the controls was noted. This was due to the fact that there was a marked crust formation, making it difficult to measure the wound edges. All the wounds were healed by Day 17. The same healing pattern occurred with C. nelsonii (Figure 3b), with the exception that the wound size increased on Day 8, rather than Day 5. In the cases of C. albopunctactum (Figure 3c) and T. sericea (Figure 3d), there was little difference in the contraction of the wounds in comparison to the controls, indicating that these two plants had little or no effect on wound contraction. The use of the 20% concentration extracts is recommended because these have a better antifungal effect (Masoko et al. 2005) and the 10% extract of T. sericea was found to have a slightly negative effect on wound healing on Day 5. All the extracts, especially C. imberbe (data not shown) were slightly better in reducing the erythema (Figure 4) of the wounds, in comparison to the controls. The exudates were slightly more prominent with the extracts (Figure 5). This could be as a result of the difficulty to distinguish between exudates and extract remnants. There was essentially no noticeable difference in crust formation between the different treatments (Figure 6). The most prominent group of biologically active compounds isolated from C. imberbe are the triterpenes (Angeh 2005). Asiaticoside is the most abundant triterpene glycoside, which is effective in wound healing and apparently acts by enhancing the induction of antioxidant levels at an early stage of wound healing (Shukla et al. 1999). In other studies, we isolated three biologically active triterpenes: terminolic, asiatic and arjunolic acids from C. nelsonii (Masoko 2006). Wound healing activity may be attributable, in part, to triterpene-rich fractions within the plant extracts we used. However, extracts from the whole plant may have better wound healing properties than a single active ingredient, possibly due to other unidentified active compounds which act synergistically (Williamson 2001). Wound contraction begins almost concurrently with collagen synthesis. The rate of contraction depends on the degree of tissue laxity and shape of the wound, with loose skin wounds and square wounds healing the fastest. Thus, rat skin, being loose, tends to heal faster than human skin, with wound contraction significantly contributing to wound closure (Stipcevic, Piljac & Piljac 2006). Consequently, wound contraction, which is usually more rapid than epithelisation, causes a decrease in the overall healing time of rat wounds (Cross et al. 2005). Although rats are not ideal for studying the efficacy of therapy on wound healing in humans due to the differences in the skin structure, they can still be used as a model for wound healing because laboratory rats are inbred and thus there is little variation in their wound healing due to genetic differences, allowing only few animals to be used (Reed et al. 1996). Laboratory rats are also relatively cheap and easy to handle and their use usually attracts less ethical objections than other animals such as pigs etc. There are also advantages in the use of rats as a research model, such as the availability of a broad knowledge based on rat wound healing and they are well established as wound models (Kimuro et al. 2005; Nayak et al. 2005; Sumitra, Manikandan & Suguna 2005). Products which have been found to improve wound healing in rats have also done so in humans. Victor-Vega et al. (2002) compared wound healing on rats and humans and found, through their experiments, that the adenosine A2A receptor antagonist significantly accelerated wound closure when applied topically. This effect was even more remarkable when compared to the recombinant human platelet-derived growth factor administered locally. Although a pig’s skin structure closely resembles that of humans, pigs were not used for practical reasons. The housing, feed and care of pigs is more expensive than other models and the psychological well-being of the pigs must be addressed by providing them with conspecific visual interaction, various toys and hand-fed treats, under professional supervision. These forms of enrichment serve to lower the distress that may otherwise be experienced and potentially confound the experimental results (Fries et al. 2005), but would be too time-consuming.
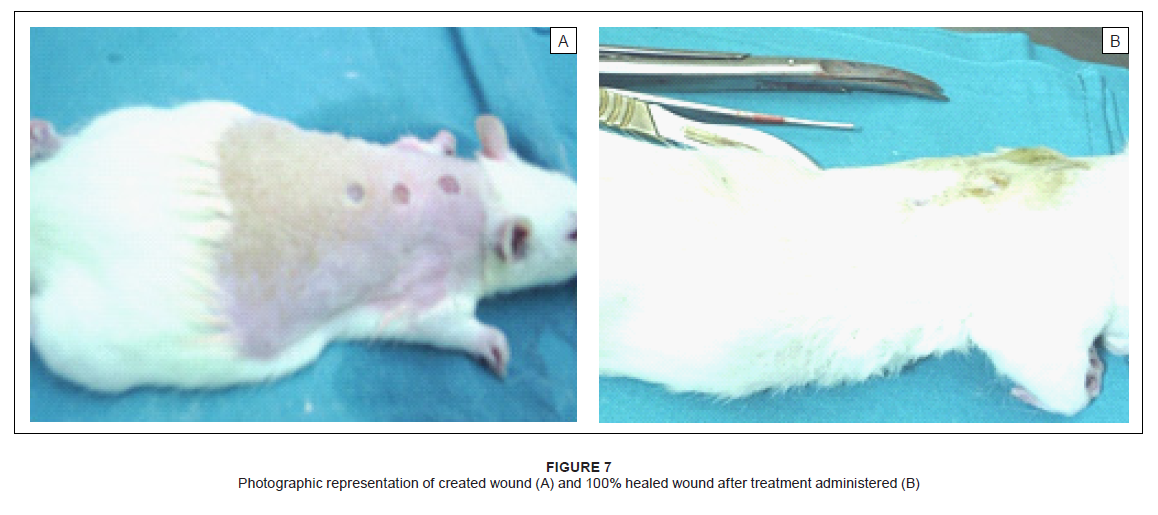 |
Figure 7: Photographic representation of created wound (A) and 100% healed wound after treatment administered (B)
|
|
|
Table 1: Evaluation of erythema and exudate
|
Our data indicate that the tested plant extracts had no adverse effects on wound healing in rats and, in fact, the acetone extracts of C. imberbe and C. nelsonii appeared to promote wound healing. This is remarkable as there was no evidence of wound infection during the trials. Although the mechanism of wound healing is unclear, our data indicate that further investigation of the Combretaceae family may be useful in this regard. Taken in conjunction with the data collected in this study and the in vitro antifungal activity of selected species, we suggest that the four selected species will also have an antifungal activity on infected wounds. We are currently assessing the effect of four selected species on rats infected with Candida albicans, Cryptococcus neoformans, Microsporum canis and Sporothrix schenckii.
We would like to acknowledge the National Research Foundation for their provision of funding, 'Mr Rudi Kotze and Mr Johan Hurter, who allowed us to collect plant material from the Lowveld National Botanical Garden, and Mr N.P. Selahle, who helped with the care of the rats and the experiment.
Angeh, J., 2005, ‘Isolation and characterization of antibacterial compounds present in members of Combretum section, Hypocrateropsis’, PhD thesis, Department of Paraclinical Sciences University of Pretoria. Anon., 2001, Guidance document on using in vitro data to estimate in vivo starting doses for acute toxicity. NIH publication number 01–4500. National Institute of Environmental Health Sciences, Research Triangle Park. Carr, J.D., 1988, Combretaceae in southern Africa, Tree Society of southern Africa, Johannesburg. Chithra, P., Sajithlal G.B. & Chandraksan, G., 1998, ‘Influence of Aloe vera Cross, S.E., Naylor, I.L., Coleman, R.A. & Teo, T., 1995, ‘An experimental model to investigate the dynamics of wound contraction’, British Journal of Plastic Surgery 48, 189–197. Eloff, J.N., 1998a, ‘Conservation of Medicinal Plants: Selecting Medicinal Plants for research and gene banking’, Monographs in Systematic Botany from the Missouri Garden 71, 209–222, in R.P. Adams & J.E. Adams, Conservation of plants Genes III: Conservation and utilisation of African plants, Missouri Botanical Garden Press, St. Louis. Eloff, J.N., 1998b, ‘Which extractant should be used for the screening and isolation of antimicrobial components from plants?’, Journal of Ethnopharmacology 60, 1–8. Eloff, J.N., 1999, ‘The antibacterial activity of 27 southern African members of the Combretaceae’, South African Journal of Science 95, 148–152. Fagbenro-Beyioku, F.A., Oyibo, W.A & Anuforom, B.C., 1998, ‘Disinfectant/antiparasitic activities of Jatropha curcas L’, East African Medicinal Journal 75, 508–511. Fries R.B., Wallace, W.A., Roy, S., Kuppusamy, P., Bergdall, V., Gordillo, G.M. et al., 2005, ‘Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen’, Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 579, 172–181. Geran, R.I., Greenberg N.H., Macdonald, M.M., Schumacher A.M. & Abbott, B.J., 1972, ‘Protocols for screening chemical agents and natural products against animal tumors and other biological systems’, Cancer Chemotherapy Reports 3, 1–17. Hutchings, A., Scott, A.H., Lewis, G. & Cunningham, A., 1996, Zulu Medicinal Plants, An Inventory, University of Natal Press: Pietermaritzburg. Inngjerdingen, K., Nergard, C.S., Diallo, D., Mounkoro, P.P. & Paulsen, B.S., 2004, ‘An ethnopharmacological survey of plants used for wound healing in Dogonland, Mali, West Africa’, Journal of Ethnopharmacology 92, 233–244. Iwu, M.M., 1993, Handbook of African Medicinal Plants, CRC Press, Florida, USA. Kimura, A., Ogata, H., Yazawa, M., Watanabe, N., Mori, T. & Nakajima, T., 2005, ‘The effects of platelet-rich plasma on cutaneous incisional wound healing in rats’, Journal of Dermatological Science 40, 205–208. Kudi, A.C., Umoh, J.U., Eduvie, L.O. & Gefu, J., 1999, ‘Screening of some Nigerian plants for antibacterial activity’, Journal of Ethnopharmacology 67, 225–228. Liu, S.Y., Sporer, F., Wink, M., Jourdane, J., Henning, R., Li, Y.L. et al., 1997, ‘Antraquinones in Rheum palmatum and Rumex dentatus (Polygonaceae), phorbol esters in Jatropha curcas L. (Euphorbiaceae) with molluscicidal activity against the schistosome vector snails, Oncomelania, Biomphalaria and Bulinus’, Tropical Medicine and International Health 2, 179–188. Mabogo, D.E.N., 1990, ‘The ethnobotany of the Vhavenda’, MSc thesis, Department of Botany University of Pretoria. Makkar, H.P., Becker K. & Schmook B., 1998, ‘Edible provenances of Jatropha curcas L. From Quintana Roo state of Mexico. The effect of roasting an antinutrient and toxic factors in seed’, Plant Foods for Human Nutrition 52, 31–36. Matsuse, I.T., Lim, Y.A., Hattori, M., Corream, M. & Gupta M.P., 1999, ‘A searh for anti-viral properties in Panamaniam plants. The effect on HIV and its essential enzymes’, Journal of Ethnopharmacology 64, 15–22. Martini, N., Katerere, D.R.P., Eloff, J.N., 2004a, ‘Antibacterial flavonoids isolated from Combretum erythrophyllum (Burch) Sond (Combretaceae)’, South African Journal of Botany 70, 310–312. Martini, N., Katerere, D.R.P. & Eloff, J.N., 2004b, ‘Biological activity of five antibacterial flavonoids isolated from Combretum erythrophyllum (Combretaceae)’, Journal of Ethnopharmacology 93, 207–212. Masoko, P., 2006, ‘Characterisation of antifungal compounds isolated from Terminalia and Combretum species (Combretaceae)’, PhD thesis, Department of Paraclinical Sciences, University of Pretoria. Masoko, P. & Eloff, J.N., 2005, ‘The diversity of antifungal compounds of six South African Terminalia species (Combretaceae) determined by bioautography’, African Journal of Biotechnology 4, 1425–1431. Masoko, P., Picard, J. & Eloff, J.N., 2005, ‘Antifungal activities of six South African Terminalia species (Combretaceae)’, Journal of Ethnopharmacology 99, 301–308. Masoko, P., Picard, J. & Eloff, J.N., 2007, ‘The antifungal activity of twenty-four South African Combretum species (Combretaceae)’, South African Journal of Botany 73, 173–183. Mcmanus, J.K.A. & Mowry, R.W., 1965, Staining Methods,Histologic and Histochemical. Harper 7 Raw, London. Mosmann, T., 1983, ‘Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays’, Journal Immunology Methods 65, 55–63. Nayak, B.S., Vinutha, B., Geetha, B. & Sudha, B., 2005, ‘Experimental evaluation of Pentas lanceolata flowers for wound healing activity in rats’, Fitoterapia 76, 671–675. Organisation for Economic Co-operation and Development, 2000, ‘Revised draft guidance document on the recognition, assessment and use of clinical signs as humane endpoints for experimental animals used in safety evaluation’, Report of the international workshop on in vitro methods for assessing acute systematic toxicity, NIH publication number 01–4499, National Institute of Environmental Health Sciences, Research Triangle Park. Oliver-Bever, B., 1986, Medicinal Plants In Tropical West Africa, Cambridge University Press, Cambridge. Priya, K.S., Gnanamani, A., Radhakrishnan, N. & Babu, M., 2002, ‘Healing potential of Datura alba on burn wounds in albino rats’, Journal of Ethnopharmacology 83, 193–199. Reed, M.J., Penn, P.E., Li, Y., Birnbaum, R., Vernon, R.B., Johnson, T.S. et al., 1996, ‘Enhanced cell proliferation and biosynthesis mediate improved wound repair in refed, caloric-restricted mice’, Mechanisms Ageing Development 89, 21–43. Simonsen, L., Petersen, M.B. & Groth, L., 1992, ‘In vivo skin penetration of salicylic compounds in hairless rats’, European Journal of Pharmaceutical Sciences 17, 95–104. Sharma, S.P., Aithal, K.S., Srinivasan, K.K., Udupa, A.L., Kumar V. & Kulkarni, D.R., 1990, ‘Anti-inflammatory and wound healing activities of the crude alcoholic extracts and flavanoids of Vitex leucoxylon’, Fitoterapia 61, 263–265. Shukla, A., Rasik, A.M., Jain, G.K., Shankar, R., Kulshrestha, D.K. & Dhawan B.N., 1999, ‘In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica’, Journal of Ethnopharmacology 65, 1–11. Spielmann, H.E., Genschow, M., Leibsch, M. & Halle, W., 1999, ‘Determination of the starting dose for acute oral toxicity. LD50 testing the up and down procedures from cytotoxicity data’, Alternatives to Laboratory Animals 27, 957–966. Staubmann, R., Ncube, I., Gubitz, G.M., Steiner W. & Read, J.S., 1999, ‘Esterase and lipase activity in Jatropha curcas L. seeds’, Journal of Biotechnology 75, 117–126. Stipcevic, T., Piljac, A. & Piljac G., 2006, ‘Enhanced healing of full-thickness burn wounds using di-rhamnolipid’, Burns 32, 24–34. Sumitra, M., Manikandan, P. & Suguna, L., 2005, ‘Efficacy of Butea monosperma on wound healing in rats’, The International Journal of Biochemistry and Cell Biology 37, 566–573. Suguma L., Chandrakasan G & Joseph K.T., 1999, ‘Influence of honey on biochemical and biophysical parameters of wounds in rats’, Journal of Clinical Biochemistry and Nutrition 14, 91–99. Suguma L., Sivakumar P. & Chandrakasan G., 1996, ‘Effects of Centella asiatica extract on dermal wound healing in rats’, Indian Journal of Experimental Biology 34, 1208–1211. Suguma, L., Surjeet, S. & Chandrakasan, G., 2002, ‘Influence of Terminalia chebula on dermal wound healing in rats’, Phytotherapy Research 16, 223–227. Tsirogianni, A.K., Moutsopoulos N.M. & Moutsopoulos H.M., 2006, ‘Wound healing: Immunological aspects’, Injury 37, 5–12. Van Wyk, B-E., Van Oudtshoorn, B. & Gericke, N., 1997, Medicinal Plants of South Africa, Briza Publications, Pretoria. Victor-Vega, C., Desai, A., Montesinos, M.C. & B.N. Cronstein, 2002, ‘Adenosine A2A receptor agonists promote more rapid wound healing than recombinant human platelet-derived growth factor (Becaplermin gel)’, Inflammation 26, 19–24. Waldorf, H. & Fewkes, J., 1995, ‘Wound healing’, Advances in Dermatology 1, 77. Williamson, E.M., 2001, ‘Synergy and other interactions in phytomedicines'. Phytomedicine 8, 401–409. Zhu S-W., Yee, B.K., Nyffeler, M., Winblad, B., Feldon, J. & Mohammed, A.H., 2006, ‘Influence of differential housing on emotional behaviour and neurotrophin levels in mice', Behavioral Brain Research 169, 10-20.
|
