|
Injury due to burning is known to impact on coagulation and haemostasis by disturbing the coagulation cascade and is also associated with impaired fibrinolysis. Also, venous thrombosis, pulmonary embolism and hypercoagulability are common during thermal injury. Using a Wistar albino rat model, we investigated in this study whether burn injury affects the ultrastructure of the fibrin networks. A typical fibrin network will contain mostly major, thick fibres with minor, thin fibres distributed amongst them. We found that the clot architecture changes after burn injury, showing more prominent minor, thin fibres in a netted appearance. Also, the clot showed areas of matted fibrin. We suggest that the thrombotic events associated with burn injury are due to the thickened and netlike areas formed when thrombin activates the coagulation cascade. This is due to impaired fibrinolysis activities, causing the resulting fibrin clots not to be successfully disseminated. Small fragments of these netted, clumped areas may therefore break loose and lead to thrombotic events after burn injuries. The current study therefore provided morphological evidence for thrombotic events associated with burn injury.>
Injury due to burning is known to impact on coagulation and haemostasis (Lavrentieva et al. 2008) by inducing subclinical disseminated intravascular coagulation. Fibrinolysis, in particular, is affected, with fibrinolytic plasma parameters showing an activation of fibrinolysis 2 hours after burn wound creation, followed by suppression in fibrinolysis after 24 hours and up to 10 days post-burn (Fang, Ding & Kong 1997). Such suppression of fibrinolysis may protect fibrin deposited in the wound, which could be important in wound healing. Fang et al. (1997) suggested that the suppressive factors of fibrinolysis may be due to enhanced activities of proteins such as plasminogen activation inhibitor-1 (PAI-1) and alpha 2-antiplasmin after injury. Also, burn injury causes an acquired deficiency of the plasma protein antithrombin III (ATIII), a natural anticoagulant that leads to a high incidence of hypercoagulability and prevalence to thrombosis in patients (Kowal-Vern et al. 2000). ATIII is a natural anticoagulant and the most important inhibitor of blood coagulation owing to its effect on thrombin (Kowal-Vern et al. 2000; Penner 1995). Venous thrombosis is associated with low plasma levels of ATIII known to occur in burn patients; also, pulmonary embolism, hypercoagulability, adult respiratory distress syndrome, infection and multiple organ failure are common in thermal injury (Darling et al. 1996; Gando, Tedo & Kubota 1992; Geerts 1994; Kowal-Vern et al. 2000; Kowal-Vern et al. 1992; Penner 1995). Ravindranath et al. (2004) also found that plasma tissue factor pathway inhibitor (TFPI) levels are significantly decreased 24 h after a burn, while thrombin activatable fibrinolytic inhibitor (TAFI) levels are significantly increased 24 h and 72 h after a burn.The question that now arises is whether burn injury has an impact on fibrin network architecture in circulating plasma, and whether a local burn injury will change the clot ultrastructure. A Wistar rat burn wound model was subsequently used to study the ultrastructure of fibrin networks.
Choice of burn wound model
Fifteen Wistar albino rats (between 200 g and 250 g each), which were maintained at the University of Pretoria Biomedical Research Centre, were used in this study (three untreated controls and 12 burn injury animals). The rats were fasted for 12 hours before thermal injury; however, they had free access to water. The rats were kept in a room with a 12-hour alternating light and dark cycle and room temperature was kept constant at 22 °C. The animals were fed a standard rat diet, provided with water ad libitum and were housed in individual polycarbonate cages. All experimental protocols complied with the requirements of the University of Pretoria Animal Use and Care Committee (Ethical Clearance number: H021/08).
Burn wound creation
On the day of burn wound creation, the rats were anaesthetised with isoflurane and directly injected with the analgesic TramadolTM. Wound creation commenced only 15 min after injection of the analgesic to allow it to take effect. The dorsum of each rat was shaved and then exposed to a 1 cm x 1 cm brass block, pre-heated to 95 °C in a hot water bath, for 10 s. The brass block was placed on the dorsum of the rats using gravity only, which resulted in partial thickness skin burns. A physiological saline solution was then administrated intraperitoneally (25 mg/kg) to prevent dehydration of the animal. Immediately after thermal injury, all wounds were dressed with a primary gauze dressing and OpsiteTM as the secondary dressing. To prevent the rats from interfering with the healing process the secondary dressing was covered with a third bandage dressing and fastened with Elastoplast around the edges. All animals received another injection of Tramadol 1 h after thermal injury. In addition, all animals received a four-hourly subcutaneous injection of analgesics for the remainder of the study.
Preparation of fibrin clots
On day 7, during termination, blood was drawn from each animal and 11 µL of citrate was added to every 100 µL of blood drawn. The blood from each animal was kept separately and studied individually. Blood was centrifuged at 1250 rpm for 2 min to obtain platelet-rich plasma (PRP).Thrombin (provided by the South African National Blood Services) was used to prepare fibrin clots (Pretorius, Ekpo & Smit 2007; Pretorius, Oberholzer & Smit 2009a; Pretorius et al. 2009b). The thrombin (20 U/mL) was prepared in a biological buffer containing 0.2% human serum albumin. When thrombin is added to PRP, fibrinogen is converted to fibrin and intracellular platelet components; for example, transforming growth factor, platelet-derived growth factor and fibroblastic growth factor are released into the coagulum. A volume of 10 µL of mouse PRP was mixed with 10 µL thrombin. The PRP–thrombin mix was immediately transferred by pipette to a 0.2-µm Millipore membrane to form the coagulum (fibrin clot). The Millipore membrane was then placed in a Petri dish on filter paper dampened with phosphate-buffered saline (PBS) (to create a humid environment) and kept at 37 °C for 10 min. The coagula-containing membranes were then washed (by placing them in PBS and magnetically stirring for 20 min) to remove any blood proteins trapped within the fibrin network.
Preparation of washed fibrin clot for scanning electron microscopy
Washed fibrin clots were fixed in 2.5% gluteraldehyde in Dulbecco’s PBS (0.075 M) at a pH of 7.4 for 1 h. Each fibrin clot was rinsed three times in phosphate buffer for 5 min before being fixed for 1 h with 1% osmium tetraoxide. The samples were rinsed three times for 5 min with distilled water and were then dehydrated serially in 30%, 50%, 70% and 90% ethanol, and three times with 100% ethanol. The scanning electron microscopy procedures were completed by drying the samples with hexamethyldisilazane (Araujo et al. 2003) before being mounted, followed by coating samples with ruthenium tetraoxide and examining the tissue with a Zeiss ULTRA Plus FEG scanning electron microscope.
Burn injury results in an inflammatory response and there is a synergistic response between the resulting inflammation and coagulation systems (Park et al. 2008; Sherwood & Toliver-Kinsky 2004). White blood cell distribution changes during the inflammatory response. As mentioned previously, haemostasis changes during burn injury. After burn injury there is a brief hypocoagulation phase, followed by hypercoagulation owing to intensified procoagulant activity. These steps, in turn, result in depression of fibrinolysis (Kirillov & Alekaeva 1975). Both thrombotic and fibrinolytic pathways are directly triggered proportionally to the extent of the burn injury (Bartlett et al. 1981; Opal 2000; Wells, Sissons & Hasleton 1984). Although this has been researched thoroughly in the past, we do not know what the impact of burn injury is on the ultrastructure of the fibrin networks. From previous research it is known that inflammation causes fibrin networks to present with a changed morphology (Oberholzer, Vieira & Pretorius 2009; Pretorius et al. 2007). Typically, control fibrin networks consist of major, thick fibres forming the bulk of the clot, with minor, thin fibres sparsely distributed among them (Pretorius et al. 2009a; Pretorius et al. 2009b). We also know that this arrangement is found in humans, rabbits and mice. However, the thickness of rodent fibrin differs considerably from that of humans (Pretorius et al. 2009b). Inflammation in humans and mice has previously been shown to look similar, although the fibre thickness varies. Pretorius and Oberholzer (2009) showed that inflammation in humans and mice show major, thick fibres covered by a thin, matted layer of minor, thin fibres. In the current study, control rat fibrin networks were studied and were found to appear similar to those of BALB/c mice; major, thick fibres with minor fibres distributed in between (Figure 1). In the burn wound injury animals in this study, fibrin fibres showed a typical inflammatory profile 7 days after injury (Figure 2a–c). Figure 2a shows major, thick fibres (thick, white arrow) and more prominent minor, thin fibres (thin, white arrows), as well as areas where fibrin has a netted appearance (black arrow). Figure 2b shows areas of the clot where thickened, matted fibrin is present (white arrow). Some areas of the clot also have a netted appearance (Figure 2c: white arrows). This changed morphology suggests that thermal injury affects not only the local area where the wound was created, but also the broader inflammatory processes and ultimately coagulation and haemostasis. Ravindranath et al. (2004) mentioned that burn injury disturbs the coagulation cascade and thrombotic process in the procoagulant pathway by impairing fibrinolysis. Also, venous thrombosis, pulmonary embolism and hypercoagulability are common in thermal injury (Darling et al. 1996; Gando et al. 1992; Geerts 1994; Kowal-Vern et al. 2000; Kowal-Vern et al. 1992; Penner 1995). Here we suggest that the thrombotic events associated with burn injury are due to the thickened, netlike areas formed when thrombin activates the coagulation cascade. Because of impaired fibrinolysis activities, the resulting fibrin clots can then not be disseminated successfully. Small fragments of these netted, clumped areas may therefore break loose and, owing to already insufficient fibrinolysis activity, cause thrombotic events after burn injuries.
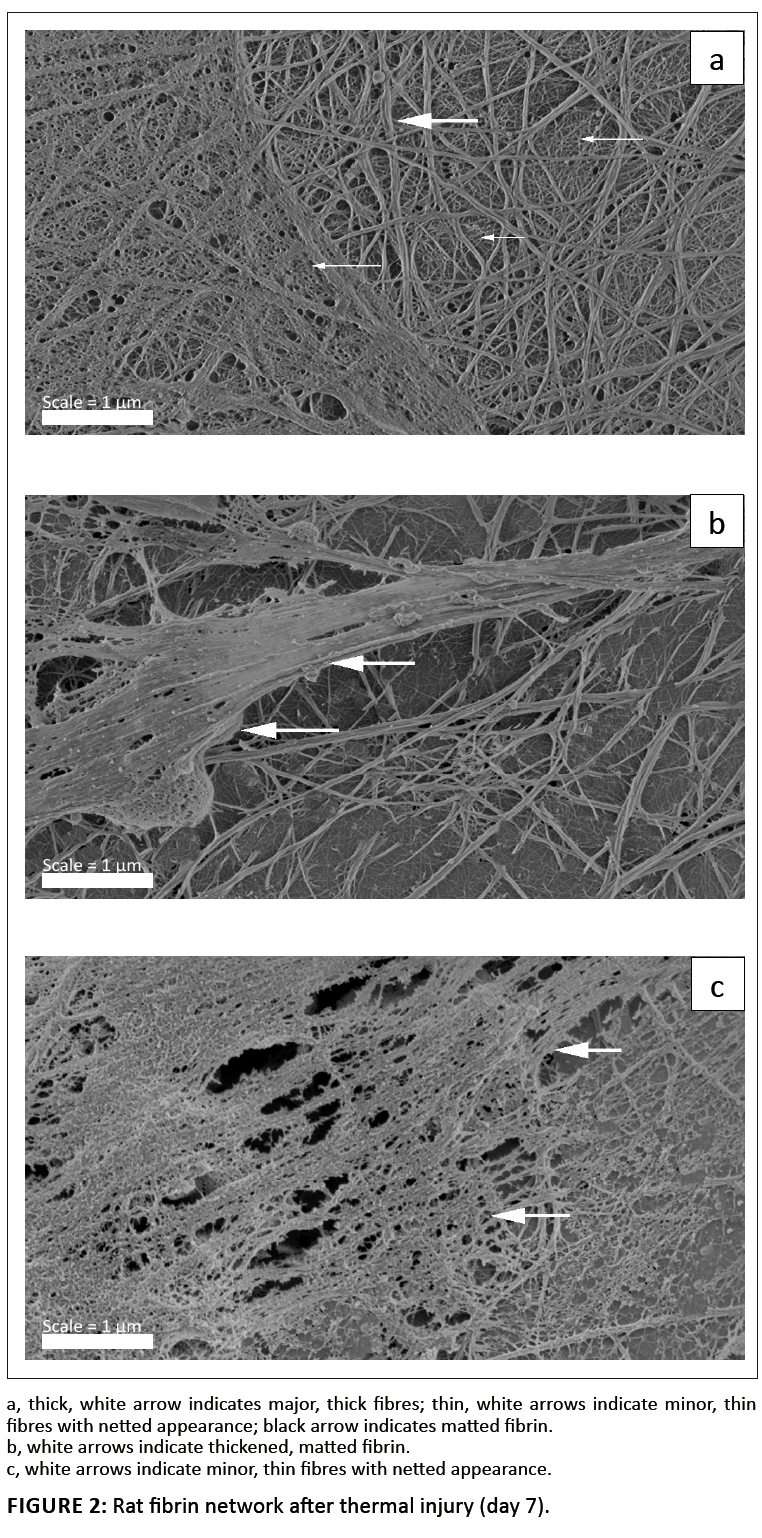 |
FIGURE 2: Rat fibrin network after thermal injury (day 7).
|
|
This study investigated whether burn injury affects the ultrastructure of fibrin networks. Results obtained with a rat burn wound model showed morphological changes to the structure of fibrin clots that formed after burn injury. These changes are likely due to impaired fibrinolysis, which explains thrombotic events associated with burn injury.
Araujo, J.C., Téran, F.C., Oliveira, R.A., Nour, E.A.A., Montenegro, M.A.P., Campos, J.R. et al., 2003, ‘Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge’, Journal of Electron Microscopy 52(4), 429–433.
doi:10.1093/jmicro/52.4.429, PMid:14599106 Bartlett, R.H., Fong, S.V., Maruggo, G., Hardeman, T. & Anderson, V., 1981, ‘Coagulation and platelets changes after thermal injury in man’, Burns 7, 370–377.
doi:10.1016/0305-4179(81)90013-9 Darling, G.E., Keresteci, M.A., Ibanez, D., Pugash, R.A., Peters, W.J. & Neligan, P.C., 1996, ‘Pulmonary complications in inhalation injuries with associated cutaneous burn’, Journal of Traumatic Injury Infection and Critical Care 40, 83–89.
doi:10.1097/00005373-199601000-00016 Fang, P., Ding, R. & Kong, L., 1997, ‘The changes of fibrinolysis in plasma and wounds in rats inflicted with deep partial thickness burns’, Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 13(4), 259–263. PMid:10452009 Gando, S., Tedo, I. & Kubota, M., 1992, ‘Post-trauma coagulation and fibrinolysis’, Critical Care Medicine 20, 594–600.
doi:10.1097/00003246-199205000-00009, PMid:1533358 Geerts, W.H., 1994, ‘Thromboembolism in trauma: the problem and its prevention’, New England Journal of Medicine 331, 1601–1606.
doi:10.1056/NEJM199412153312401, PMid:7969340 Kirillov, M.M. & Alekaeva, L.D., 1975, ‘Changes in the blood coagulation system in patients with mechanical trauma and burns’, Problemy Gematologii i Perelivaniia Krovi 20(6), 15–18. PMid:1135183 Kowal-Vern, A., McGill, V., Walenga, J.M. & Gamelli, R.L., 2000, ‘Antithrombin III concentrate in the acute phase of thermal injury’, Burns 26(1), 97–101.
doi:10.1016/S0305-4179(99)00099-6 Kowal-Vern, A., Walenga, J.M., Gamelli, R.L., Hoppensteadt, D., Sharp-Pucci, M.M. & Schumacher, H.R., 1992, ‘The effect of burn wound size on hemostasis: a correlation of the hemostatic changes to the clinical state’, Journal of Traumatic Injury Infection and Critical Care 33, 50–56.
doi:10.1097/00005373-199207000-00011 Lavrentieva, A., Kontakiotis, T., Bitzani, M., Papaioannou-Gaki, G., Parlapani, A., Thomareis, O. et al., 2008, ‘Early coagulation disorders after severe burn injury: impact on mortality’, Intensive Care Medicine 34(4), 700–706.
doi:10.1007/s00134-007-0976-5, PMid:18193192 Oberholzer, H.M., Vieira, W.A. & Pretorius, E., 2009, ‘Investigating the effect of the homeopathic immunomodulator, MODUL8®, on blood count, bronchial lavage and fibrin ultrastructure on experimental asthmatic BALB/c mice’, International Journal of Morphology 27(3), 955–963. Opal, S.M., 2000, ‘Phylogenetic and functional relationships between coagulation and the innate immune response’, Critical Care Medicine 28(9), 77–80.
doi:10.1097/00003246-200009001-00017, PMid:11007204 Park, M.S., Salinas, J., Wade, C.E., Wang, J., Martini, W., Pusateri, A.E. et al., 2008, ‘Combining early coagulation and inflammatory status improves prediction of mortality in burned
and non-burned trauma patients’, Journal of Trauma 64(2), 188–194.
doi:10.1097/TA.0b013e318160a5a3, PMid:18376164 Penner, J., 1995, ‘Antithrombin deficiency in special clinical syndromes – part II: trauma/burns,’ Seminars in Hematology 32, 42–48. PMid:8821209 Pretorius, E. & Oberholzer, H.M., 2009, ‘Ultrastructural changes of platelets and fibrin networks in human asthma: a qualitative case study’, Blood Coagulation and Fibrinolysis 20(2), 146–149.
doi:10.1097/MBC.0b013e328325549a Pretorius, E., Ekpo, O.E. & Smit, E., 2007, ‘Comparative ultrastructural analyses of platelets and fibrin networks using the murine model of asthma’, Experimental and Toxicologic Pathology 59(2), 105–114.
doi:10.1016/j.etp.2007.02.011, PMid:17600694 Pretorius, E., Oberholzer, H.M. & Smit, E., 2009a, ‘Ultrastructure of platelets and fibrin networks of asthmatic mice exposed to selenium and Withania somnifera’, Anatomical Science International 84(3), 210–217.
doi:10.1007/s12565-008-0010-1, PMid:19214657 Pretorius, E., Vieira, W.A., Oberholzer, N. & Auer, R.E.J., 2009b, ‘Comparative scanning electron microscopy of platelets and fibrin networks of humans and different animals’, International Journal of Morphology 27(1), 69–76. Ravindranath, T.M., Goto, M., Demir, M., Tobu, M., Kujawski, M.F., Hoppensteadt, D. et al., 2004, ‘Tissue factor pathway inhibitor and thrombin activatable fibrinolytic inhibitor
plasma levels following burn and septic injuries in rats’, Clinical and Applied Thrombosis/Hemostasis 10, 379–385.
doi:10.1177/107602960401000411, PMid:15497025 Sherwood, E.R. & Toliver-Kinsky, T., 2004, ‘Mechanisms of the inflammatory response’, Best Practice and Research: Clinical Anaesthesiology 18(3), 385–405.
doi:10.1016/jbpa.2003.12.002 Wells, S., Sissons, M. & Hasleton, P.S., 1984, ‘Quantitation of pulmonary megakaryocytes and fibrin thrombi in patients dying from burns’, Histopathology 8, 517–527.
doi:10.1111/j.1365-2559.1984.tb02361.x, PMid:6735362
|